Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Hideya; Tsubata, Yasuhiro; Kurosawa, Tatsuya*; Sagawa, Hiroshi*; Matsumura, Tatsuro
Journal of Nuclear Science and Technology, 54(11), p.1163 - 1167, 2017/11
Times Cited Count:26 Percentile:93.1(Nuclear Science & Technology)A highly practical diamide-type extractant, which is an alkyl diamide amine with 2-ethylhexyl alkyl chains (ADAAM(EH)), was investigated for mutual separation of Am(III) and Cm(III). ADAAM(EH) is a multidentate ligand with one soft N-donor atom and two hard O-donor atoms in its central frame. This tridentate arrangement of donor atoms provides selective binding to Am(III) compared to that with Cm(III) in highly acidic media, resulting in separation factors of up to 5.5. A continuous liquid-liquid extraction and stripping test was conducted using a multistage countercurrent mixer-settler extractor with ADAAM(EH) in n-dodecane. In this test, separation of Am(III) and Cm(III) was achieved with very high yield.
Sasaki, Yuji; Morita, Keisuke; Saeki, Morihisa*; Hisamatsu, Shugo; Yoshizuka, Kazuharu*
Hydrometallurgy, 169, p.576 - 584, 2017/05
Times Cited Count:14 Percentile:55.94(Metallurgy & Metallurgical Engineering)The novel tridentate extractant including soft donor has developed and examined. The extractant, tetraoctyl-thiodiglycolamide (TDGA), is analogous structure to tetraoctyl-diglycolamide (TODGA) and methylimino-dioctylacetamide (MIDOA), but with sulfur donor instead of ether oxygen or nitrogen atoms of TODGA or MIDOA. From the present work, TDGA can extract silver, palladium, gold, and mercury from acidic solutions to n-dodecane. In addition to these results, the distribution ratios of hard and soft acid metals by using TDGA, TODGA, and MIDOA are compared, where the metal-complexations with each donor atom are investigated. 1H-NMR and IR studies for the metal-TDGA complexes indicate the role on donor atoms, S and N, of TDGA.
Suzuki, Hideya; Tsubata, Yasuhiro; Kurosawa, Tatsuya; Shibata, Mitsunobu; Kawasaki, Tomohiro; Urabe, Shunichi*; Matsumura, Tatsuro
Analytical Sciences, 32(4), p.477 - 479, 2016/04
Times Cited Count:24 Percentile:68.22(Chemistry, Analytical)An impeccable, high-performance new reagent called alkyl diamide amine (ADAAM) was examined from the viewpoint of mutual separation of Am(III) and Eu(III). ADAAM has three donor atoms, one soft N-donor atom and two hard O-donor atoms, in the central frame. The combination of soft and hard atoms affords a tridentate donor set of atoms that ensures remarkable extractability and selectivity of Am(III) and Eu(III) in highly acidic media.
Matsumura, Tatsuro; Takeshita, Kenji*
ACS Symposium Series, 933, p.261 - 273, 2006/07
Three TPEN isomers with different positon of nitrogen donor in pyridyl groups, t2pen, t3pen and t4pen, were synthesized and the extraction separation of Am(III) and Eu(III) with these ligands and a fatty acid, decanoic acid, was investigated. All isomers were similar in the complexation in the aqueous phase, such as the protonation and the formation of metal complex, however, they showed different extraction behavior of Am and Eu. The synergistic extraction effect for Am was observed for t2pen and the high separation factor about 100 was measured, when 1:2. The value is comparable to that for the extraction system with a famous nitrogen-donor extractant, BTP. On the other hand, the extractability of other isomers was very low and no separation of Am and Eu was observed. Only t2pen, in which nitrogen donor in pyridyl groups is positioned in the vicinity of the skeletal structure (N-C-C-N structure) of ligand, is available for the extraction separation of Am.
Mirvaliev, R.*; Watanabe, Masayuki; Matsumura, Tatsuro; Tachimori, Shoichi*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 41(11), p.1122 - 1124, 2004/11
Times Cited Count:21 Percentile:76.47(Nuclear Science & Technology)Transmutation is a technology aimed to reduce HLW from reprocessing process. Minor actinides in the HLW will be converted to short-lived nuclides. However, lanthanides in HLW adversely affects on the efficiency of the transmutation. It is well known that separating An(III) and Ln(III) is very difficult because of their similarity of chemical properties. Therefore, the separation is one of the essential subjects to establish the transmutation technology. Considerable efforts have been devoted to the development of new extractants for the separation. N,N,N',N'-tetrakis(2-methylpyridyl)ethylenediamine (TPEN) demonstrates 100-fold preference for Am(III) over Ln(III) between stability constants with the ions in the aqueous phase. We have reported that Am(III) was selectively extracted from the aqueous phase containing Ln(III) by TPEN in nitrobenzene system and synergistic system with TPEN and D2EHPA in octanol. This work presents our recent results that Am(III) is separated from Eu(III) by a synergistic extraction system with TPEN and decanoic acid diluted with 1-octanol.
 solutions using
solutions using 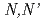 -dimethyl-
-dimethyl-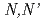 -diphenylpyridine-2,6-dicarboxyamide
-diphenylpyridine-2,6-dicarboxyamideShimada, Asako; Yaita, Tsuyoshi; Narita, Hirokazu; Tachimori, Shoichi; Kimura, Takaumi; Okuno, Kenji*; *
Solvent Extraction Research and Development, Japan, 11, p.1 - 10, 2004/04
The distribution ratio (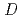 ) of Am(III) and lighter Ln(III) in the extraction with
) of Am(III) and lighter Ln(III) in the extraction with 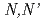 -dimethyl-
-dimethyl-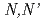 -diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO
-diphenylpyridine-2,6-dicarboxyamide(DMDPhPDA) from HNO solutions were determined. The D's increased with an increase of HNO
solutions were determined. The D's increased with an increase of HNO concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO
concentration. Additionally, separation of Am(III) from Ln(III) were confirmed for all the HNO concentration range (S.F.=(D
concentration range (S.F.=(D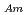 /D
/D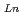 )). From 4 M HNO
)). From 4 M HNO solution with 0.5 M DMDPhPDA CHCl
solution with 0.5 M DMDPhPDA CHCl solution, the
solution, the 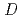 's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO
's of Am(III), Eu(III) and Nd(III) were 1.3, 0.25 and 0.24, respectively. This result suggests that Am(III) can be separated from Eu and Nd in the higher HNO concentration region than that has been reported so far. These
concentration region than that has been reported so far. These 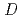 's value and the S.F. were reproduced in the extraction from the metal concentration range from 10
's value and the S.F. were reproduced in the extraction from the metal concentration range from 10 M order to 10
M order to 10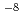 M.
M.
Tsuchida, Yusuke; Matsumiya, Masahiko*; Sasaki, Yuji; Akama, Takeshi*; Arita, Yuji*
no journal, ,
We study the solvent extraction of soft acid metals, e.g., platinum metals, gold and silver from nitric and hydrochloric acids by commercially available organic compounds with low solubility in water. We would like to know some of the physical parameters obtained from DFT calculations can support the experimental results. The present work show that the extractants having S-donor give high extractability for gold.